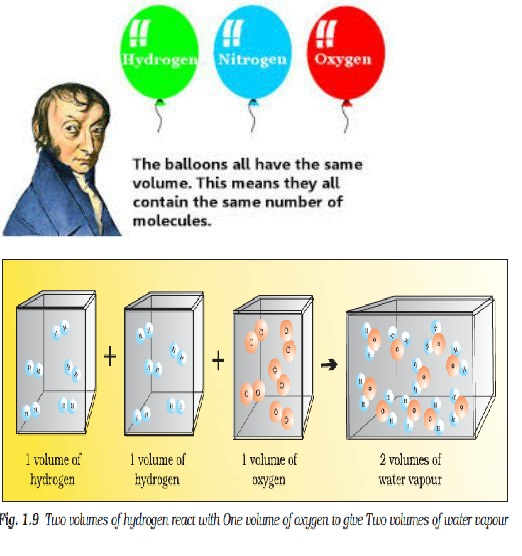
Law of Conservation of Mass :
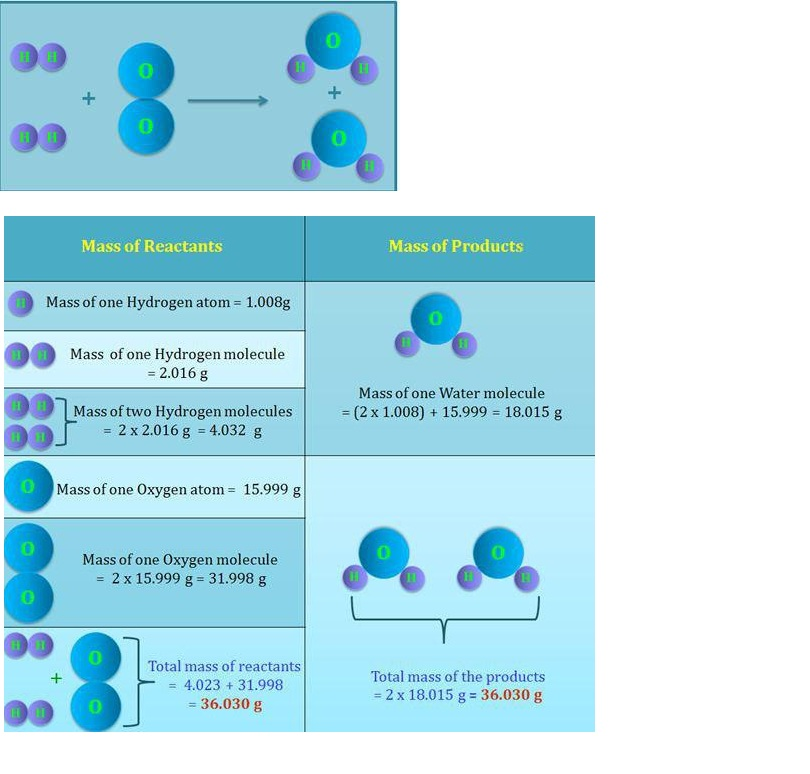
Statement : Matter can neither be created nor destroyed.
Antoine Lavoisier (1789) gave this law based on his experimental studies for combustion reactions.
1.7 gram of silver nitrate dissolved in 100 gram of water is taken. 0.585 gram of sodium chloride
dissolved in I 00 gram of water is added to it and chemical reaction occurs. 1.435 gm of AgCI and 0.85
gm `NaN0_3` are formed. Show that these results illustrate the Jaw of conservation of mass.
Total mass before chemical change = mass of`AgN0_3` + Mass of NaCJ + Mass of water
= 1.70 + 0.585 + 200 = 202.285 gram
Total mass after the chemical reaction = mass ofAgCI + Mass of`NaN0_3` + Mass of water
= 1.435 + 0.85 + 200 = 202.285 gram
Thus in the given reaction
Total mass of reactants = Total mass of the products.
Antoine Lavoisier (1789) gave this law based on his experimental studies for combustion reactions.
1.7 gram of silver nitrate dissolved in 100 gram of water is taken. 0.585 gram of sodium chloride
dissolved in I 00 gram of water is added to it and chemical reaction occurs. 1.435 gm of AgCI and 0.85
gm `NaN0_3` are formed. Show that these results illustrate the Jaw of conservation of mass.
Total mass before chemical change = mass of`AgN0_3` + Mass of NaCJ + Mass of water
= 1.70 + 0.585 + 200 = 202.285 gram
Total mass after the chemical reaction = mass ofAgCI + Mass of`NaN0_3` + Mass of water
= 1.435 + 0.85 + 200 = 202.285 gram
Thus in the given reaction
Total mass of reactants = Total mass of the products.